 Alzheimer disease (AD) and type 2 diabetes mellitus (T2D) are characterized by the presence of insoluble fibril deposits due to misfolded proteins (amyloids).
Alzheimer disease (AD) and type 2 diabetes mellitus (T2D) are characterized by the presence of insoluble fibril deposits due to misfolded proteins (amyloids).
A new study entitled “In Vivo Seeding and Cross-Seeding of Localized Amyloidosis- A Molecular Link between Type 2 Diabetes and Alzheimer Disease” was published in The American Journal of Pathology by Marie E. Oskarsson part of Dr. Gunilla T. Westermark’s group from the Department of Medical Cell Biology at Uppsala University in Sweden along with colleagues. This work suggests brain amyloid can stimulate the growth of fibrils in mouse pancreas and that pancreatic-related amyloid was found to be associated with brain-related amyloid in human brain senile plaques.
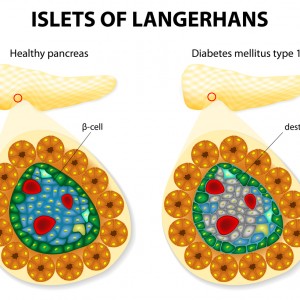
The majority of patients with type 2 diabetes mellitus have islet amyloid in islets of Langerhans. The islet amyloid is composed of amyloid polypeptide (IAPP), which is derived from its precursor proIAPP and the IAPP accumulation may lead to beta-cell death. In patients with Alzheimer’s disease, deposits of beta-amyloid in the brain cortex and blood vessels are frequently observed.
Many clinical studies have demonstrated that individuals with T2D have almost twice the probability of developing AD. The present study suggests an association between T2D and AD that may be due to the processes that induce amyloidosis. The main goal of this research was to understand the mechanism by which the amyloid deposits start to form, multiply in tissue or move from one organ to another.
Dr. Westermark said in a news release that structures that have the potential to form amyloids may induce either homologous or heterologous fibril growth, i.e. fibril growth from the same or different amyloid protein. Dr. Westermark added that heterologous formation between IAPP and beta-amyloid may be the molecular link between AD and T2D.
To test this hypothesis, the researchers administered preformed fibrils of synthetic IAPP, proIAPP, or beta-amyloid to transgenic mice expressing human IAPP. They then fed the mice a high-fat diet for 10 months. The brain and pancreas tissues were then analyzed using a dye specific for amyloid. The number of islets with amyloid were considerably augmented with all three types of fibrils when compared to controls. There were no amyloid deposits in other organs. These findings have shown that fibril injections could seed amyloid deposits in the pancreas and that amyloid from the brain could cross-seed fibril formation in the pancreas.
Additionally, the researchers analyzed human tissues from the pancreas and brain from patients diagnosed with T2D and found that pancreas sections with islet amyloid contained IAPP.
Dr. Westermark said that it was not possible to prove that IAPP in the brain was brain-derived or came from pancreatic beta-cells. “Cross-seeding by other amyloid aggregates or perhaps by other types of aggregates offers one possible mechanism for initiation of amyloid formation,” added Dr. Westermark.
Finally, Dr. Westermark concluded that interactions between amyloid and other proteins that are susceptible to form aggregates may be crucial to the development of diseases that are associated to protein-misfolding.