The American Diabetes Association and the European Association for the Study of Diabetes are alerting the community of diabetes patients and the medical community about the lack of information on the safety and efficacy of insulin pumps. The warnings are to be published in the April issue of the Diabetes Care journal and are the result of a joint effort between the two associations, which advocate for more information to ensure that health care providers are able to educate diabetic patients.
The American Diabetes Association and the European Association for the Study of Diabetes are alerting the community of diabetes patients and the medical community about the lack of information on the safety and efficacy of insulin pumps. The warnings are to be published in the April issue of the Diabetes Care journal and are the result of a joint effort between the two associations, which advocate for more information to ensure that health care providers are able to educate diabetic patients.
The statement is comprised of a series of recommendations based on the belief that there is a need for a comprehensive safety overhaul of information provided by pump producers and publicly funded research about insulin pumps. The organizations aim to encourage “the adoption of a more rigorous, standardized and transparent approach to safety.”
The main goal of the initiative is to ask for standardized regulatory procedures, since the U.S. Food and Drug Administration (FDA) and the European Union (EU) have different revision regulations for pump manufacturing. They recommend the use of a single, international and publicly accessible database that includes adverse events, as well as open information on products, study outcomes, and design features, according to a press release.
“Technology is evolving rapidly for treating diabetes,” stated the director of the University of Southern California Clinical Diabetes Program, Anne Peters, MD, who co-authored the statement. “While that’s certainly a good thing, we don’t have very good post-marketing surveillance for devices such as insulin pumps, particularly in Europe where manufacturers often introduce products prior to releasing them in the United States. We need to make sure we have sufficient data about how the devices are working once they hit the market, so that we can support patients by helping them understand how to prevent errors in using them.”
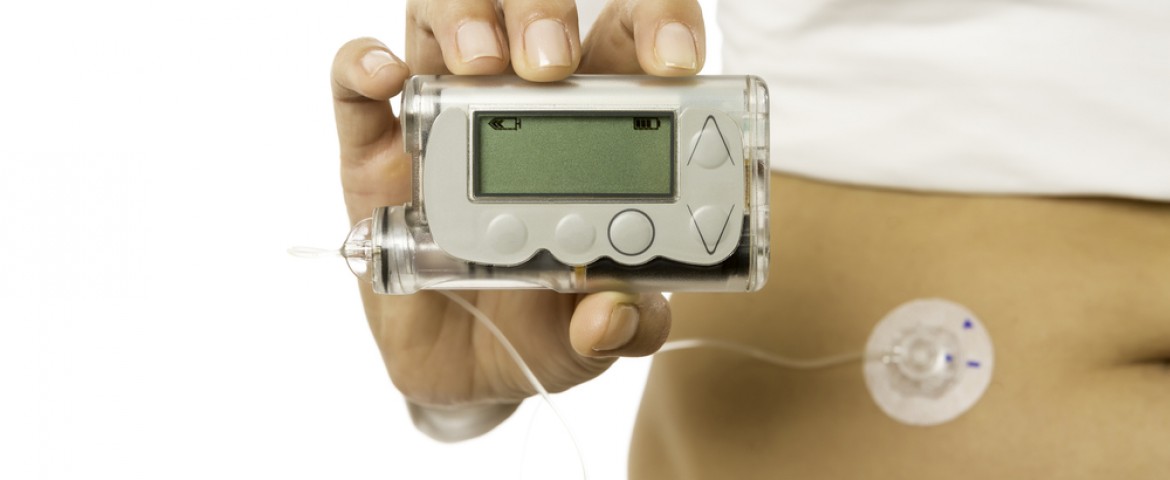
Over 200 million people around the world suffer from diabetes, and since most of the patients with type 1 diabetes need to use insulin pumps and some with type 2 diabetes as well, the device is used by approximately one million patients. However, there is always the problem that the device can break and patients will need to wait days to replace it, according to Peters. Therefore, she also advises patients to have a device failure plan, including a long-acting type of insulin that starts to lower blood glucose within 4 to 6 hours after injection and with the strongest effect 10 to 18 hours after injection.