The U.S. Food and Drug Administration (FDA) recently published an article on its Consumer Updates page, which recommends continuous glucose monitors (CGMs) and insulin pumps for patients suffering from diabetes who need to control their insulin levels regularly. The FDA also noted the importance of managing blood sugar levels in order to improve health and prevent other serious associated conditions.
CGMs and insulin pumps may help diabetes patients control and stabilize their normally unstable glucose levels, which are caused by a malfunction of the pancreas. Poorly managed blood glucose can lead to life-altering and even life-threatening complications of diabetes, such as heart attacks, stroke, kidney diseases, nerve damages, loss of toes or feet, digestive problems, blindness, gum problems and loss of teeth.
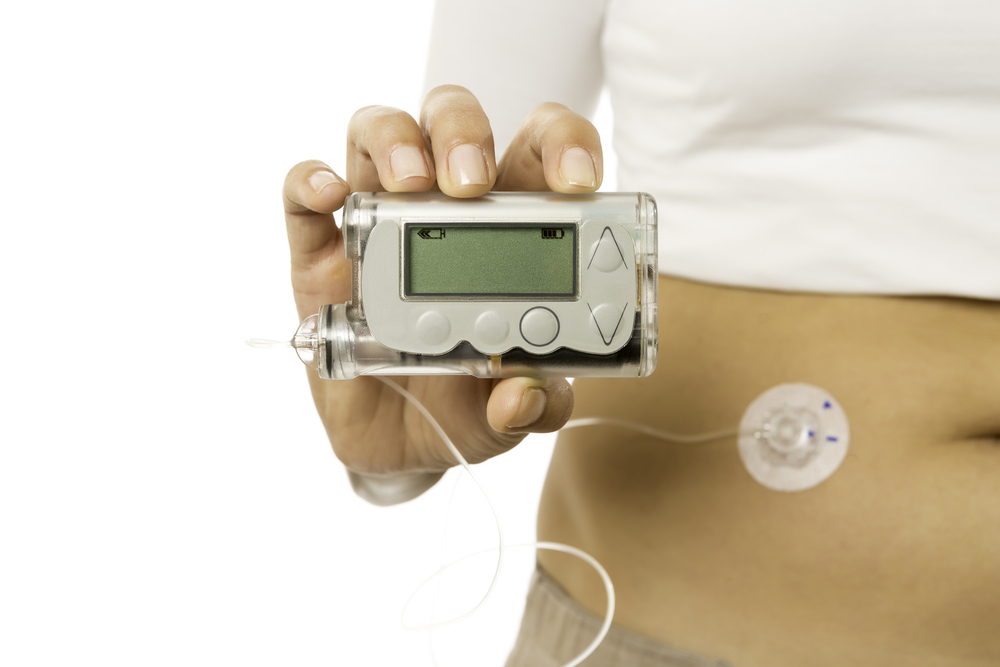
The insulin pump is a computerized device that is able to deliver a steady flow of insulin during the sleep. There are several FDA approved insulin pumps, which are the size of pagers and are connected to a tube outside the body. The CGMs measure the glucose with sensors every five minutes, and wirelessly transmits the readings to a receiver. However, the FDA reminds that there has yet to be a ruling stating the use of CGM values alone as a definitive measure of determining insulin dosage.
“These devices are an important technological advance to address some of the challenges people with diabetes face in managing their blood sugar,” explained the director of FDA’s Office of In Vitro Diagnostics and Radiological Health, Alberto Gutierrez, Ph.D. “As they become better integrated with insulin pumps, CGMs can ease the daily burden of people with diabetes who juggle the use of multiple medical devices.”
Currently, the Medtronic MiniMed and the Animas Vibe System are two FDA-approved, CGM-enabled insulin pumps. The device from Medtronic has been on the American market since April 2006, while the latter was approved last November, 25, 2014, and is the combination of the DexCom G4 Platinum CGM and an Animas insulin pump.
The FDA report includes an update on an emerging method for managing diabetes, called, the artificial pancreas device system (APDS). There are investigators dedicated to the development of these automated, closed-loop systems that aim to provide a combination of a continuous glucose monitor, an insulin infusion pump, and a “smart” system in order to improve the monitoring of glucose levels, automatically pump the appropriate dosage of insulin, and reduce the impact on patients.
“The ideal APDS will be a system of devices that closely mimics the glucose-regulating function of a healthy pancreas,” explains the FDA article. “It will not only monitor glucose levels but also automatically adjust the delivery of insulin to reduce high blood glucose levels (hyperglycemia) and minimize the incidence of low blood glucose (hypoglycemia).”