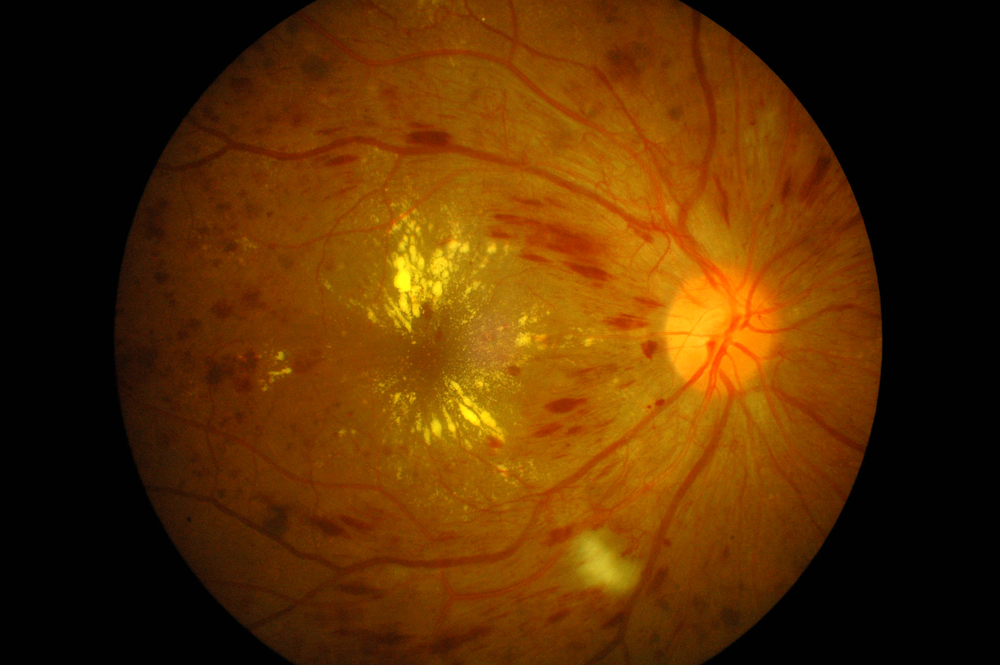
The U.S. Food and Drug Administration on Wednesday, March 25 expanded the approved use for Eylea (aflibercept) injections for treatment of diabetic retinopathy in patients with diabetic macular edema.
According to the National Institutes of Health (NIH), diabetic retinopathy (DR), an ocular manifestation of diabetes caused by changes in retinal blood vessels, is the most common eye disease in people with diabetes and the leading cause of new blindness among U.S. adults aged 20 to 74. In the United States, diabetic retinopathy accounts for 12 percent of all new blindness cases annually. In 2008, 33 percent of adults with diabetes aged 40 years or older had some form of DR.
The Centers for Disease Control and Prevention (CDC) estimates that diabetes (including both type 1 and type 2) affects more than 29 million people in the United States.
In some diabetic retinopathy cases, blood vessels may swell and leak fluid, while other diabetic individuals may present with abnormal new blood vessels growing on the surface of the retina — the light-sensitive tissue at the back of the eye on which the lens projects images for transfer to the brain. Retina dysfunctions are antagonistic to good vision, severe vision loss or blindness can occur if the new DR-related blood vessels break.
Persons with diabetic retinopathy may not notice changes to their vision initially, but over time, diabetic retinopathy, which usually affects both eyes, tends to worsen and can cause vision loss. The longer a person has diabetes, the greater likelihood of their developing diabetic retinopathy, and the condition affects up to 80 percent of all patients who have lived with diabetes for 10 years or longer. However, research suggests that at the devastating effects of least 90 percent of these new cases could be reduced if infrastructure for proper and vigilant treatment and monitoring of the eyes was in place.
 “Diabetes is a serious public health crisis, affecting more patients every year,” says Edward Cox, M.D., M.P.H, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research, in a FDA release. “Today’s approval gives patients with diabetic retinopathy and diabetic macular edema another therapy to treat this vision-impairing complication.”
“Diabetes is a serious public health crisis, affecting more patients every year,” says Edward Cox, M.D., M.P.H, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research, in a FDA release. “Today’s approval gives patients with diabetic retinopathy and diabetic macular edema another therapy to treat this vision-impairing complication.”
In February, the FDA approved Lucentis (ranibizumab injection) 0.3 mg to treat DR in patients with DME. Lucentis is marketed by South San Francisco, California-based Genentech, a subsidiary of Roche Pharmaceuticals.
EYLEA (aflibercept) Injection, marketed by Tarrytown, N.Y.-based Regeneron Pharmaceuticals Inc., is a prescription medication administered by ocular injection, and approved for treatment of patients with:
• Diabetic Macular Edema (DME) and Diabetic Retinopathy (DR) in patients with DME: The recommended dose for EYLEA is 2 mg administered by injection in the eye every 2 months (8 weeks) following 5 initial monthly (4 weeks) injections. EYLEA may be dosed once per month, but additional benefit was not seen with this dosing plan.
• Wet Age-related Macular Degeneration (AMD): The recommended dose for EYLEA is 2 mg administered by injection in the eye every 2 months (8 weeks) following 3 initial monthly (4 weeks) injections. EYLEA may be dosed once per month, but additional benefit was not seen with this dosing plan.
• Macular Edema following Retinal Vein Occlusion (RVO): The recommended dose for EYLEA is 2 mg administered by injection in the eye monthly (every 4 weeks).
Eylea therapy involves a physician injecting the drug into the eye once per month for the first five injections, and thereafter once every second month. The medication is intended for use in conjunction with appropriate interventions to control blood sugar, blood pressure and cholesterol.
Eylea’s safety and efficacy in treating DR in patients with DME were evaluated in 679 participants in two clinical studies in which participants were randomly assigned to receive either Eylea or macular laser photocoagulation, which is a laser-based treatment used to burn small areas of the retina. At week 100 of the trial, participants receiving the Eylea treatment were typically experiencing with significant improvement in DR severity in comparison with patients not receiving Eylea.
Side effects most commonly associated with Eylea therapy include bleeding of the conjunctiva (the transparent filmy tissue lining the inside of the eyelids and covering the white area of the eye); ocular pain; cataracts; increased floaters; increased pressure inside the eye (increased intraocular pressure); and separation of the interior jelly of the eye from the retina (vitreous detachment). Serious adverse reactions include infection within the eye (endophthalmitis) and detached retinas.
In the case of Eylea, the FDA exercised its power to designate a drug a “breakthrough therapy” at the sponsor’s request provided that preliminary clinical evidence indicates the drug may be a substantial improvement over available therapies for patients with serious or life-threatening conditions. Breakthrough therapy designation has been granted to Eylea for DR with DME treatment of. The FDA has also reviewed the new use for Eylea under the agency’s priority review program that allows expedited review of drugs demonstrating potential for significant safety or effectiveness advances in treatment of serious conditions.
The FDA had previously approved Eylea to treat wet (neovascular) age-related macular degeneration, a condition in which abnormal blood vessels grow and leak fluid into the macula. Eylea is also approved to treat DME and macular edema secondary to retinal vein occlusions, both of which cause fluid to leak into the macula resulting in blurred vision.
Sources:
U.S. Food and Drug Administration
Regeneron Pharmaceuticals Inc.
National Institutes of Health (NIH)
Centers for Disease Control and Prevention (CDC)
Image credit:
U.S. Food and Drug Administration