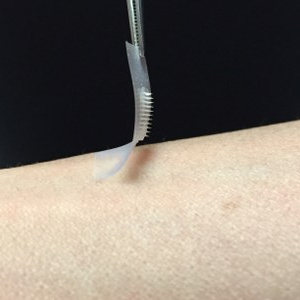
The pain and tedium associated with daily insulin injections could soon be passé for the millions of Americans living with diabetes, thanks to a new “smart” insulin transdermal patch invented by researchers at the University of North Carolina and NC State. The smart patch can detect blood sugar level spikes and secrete doses of insulin into the bloodstream whenever imbalances occur.
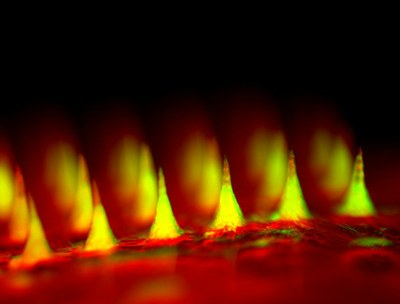
The patch device, which occupies a thin square of dermal real estate no bigger than a penny is covered with more than one hundred tiny needles, each about the size of an eyelash. These microneedles are packed with microscopic storage units for insulin and glucose-sensing enzymes that trigger them to rapidly release their cargo when blood sugar levels get too high.
A study by the patch’s developers published in the Proceedings of the National Academy of Sciences (PNAS), found that the painless device could lower blood glucose in a mouse model of type 1 diabetes for up to nine hours. The researchers caution that more pre-clinical test and subsequent clinical trials in humans will be required before the patch can be administered to patients, but affirm that the innovation shows great promise.
The PNAS study, entitled “Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery“ (doi: 10.1073/pnas.1505405112), is coauthored by Jicheng Yu, Yuqi Zhang, Yanqi Ye, Rocco DiSanto, Wujin Sun, Davis Ranson, Frances S. Ligler, John B. Buse, and Zhen Gu, variously of the Joint Department of Biomedical Engineering of the University of North Carolina at Chapel Hill and North Carolina State University at Raleigh, NC; the Molecular Pharmaceutics Division and Center for Nanotechnology in Drug Delivery Eshelman School of Pharmacy of the University of North Carolina at Chapel Hill NC; and the University of North Carolina School of Medicine at Chapel Hill.
The researchers note that in exploiting synthetic glucose-responsive insulin delivery systems, challenges remain to demonstrate a strategy that would combine 1) fast responsiveness, 2) ease of administration, and 3) excellent biocompatibility. They report that they have developed a novel glucose-responsive insulin delivery device using a painless microneedle-array patch containing hypoxia-sensitive hyaluronic acid-based vesicles.
The study explains that these vesicles quickly dissociate and release encapsulated insulin under the local hypoxic environment, caused by the enzymatic oxidation of glucose in the hyperglycemic state. This smart insulin patch with a new enzyme-based glucose-responsive mechanism can regulate the blood glucose of type 1 diabetic mice to achieve normal levels, with faster responsiveness compared with the commonly used pH-sensitive formulations, and can avoid the risk of hypoglycemia.
The coauthors observe that a glucose-responsive closed-loop insulin delivery system mimicking the function of pancreatic cells has tremendous potential to improve diabetics’ quality of life and health. Resulting from their research is a novel glucose-responsive insulin delivery device that uses a painless microneedle-array patch (ie: “smart insulin patch”) containing glucose-responsive vesicles (GRVs; with an average diameter of 118 nm), which are loaded with insulin and glucose oxidase (GOx) enzyme. The local hypoxic microenvironment caused by the enzymatic oxidation of glucose in the hyperglycemic state promotes the reduction of HS-HA, which in turn rapidly triggers the dissociation of vesicles and subsequent release of insulin. The smart insulin patch effectively regulated the blood glucose in a mouse model of chemically induced type 1 diabetes.
The coauthors say the work described in the PNAS paper is the first demonstration, to their knowledge, of a synthetic glucose-responsive device using a hypoxia trigger for regulation of insulin release. They observe that the faster responsiveness of this approach holds promise in avoiding hyperglycemia and hypoglycemia if translated for human therapy.
This article also contains supporting information online at http://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505405112/-/DCSupplemental
 “We have designed a patch for diabetes that works fast, is easy to use, and is made from nontoxic, biocompatible materials,” says paper co-senior author Zhen Gu, PhD, a professor in the Joint UNC/NC State Department of Biomedical Engineering. Dr. Gu also holds appointments in the UNC School of Medicine, the UNC Eshelman School of Pharmacy, and the UNC Diabetes Care Center. “The whole system can be personalized to account for a diabetics weight and sensitivity to insulin,” Dr. Gu adds, “so we could make the smart patch even smarter.”
“We have designed a patch for diabetes that works fast, is easy to use, and is made from nontoxic, biocompatible materials,” says paper co-senior author Zhen Gu, PhD, a professor in the Joint UNC/NC State Department of Biomedical Engineering. Dr. Gu also holds appointments in the UNC School of Medicine, the UNC Eshelman School of Pharmacy, and the UNC Diabetes Care Center. “The whole system can be personalized to account for a diabetics weight and sensitivity to insulin,” Dr. Gu adds, “so we could make the smart patch even smarter.”
Diabetes is estimated to affect more than 387 million people worldwide, with that number is expected to grow to 592 million by the year 2035.  “Patients with type 1 and advanced type 2 diabetes try to keep their blood sugar levels under control with regular finger pricks and repeated insulin shots, a process that is painful and imprecise,” says John Buse, MD, PhD, co-senior author of the PNAS paper and director of the UNC Diabetes Care Center, adding: “Injecting the wrong amount of medication can lead to significant complications like blindness and limb amputations, or even more disastrous consequences such as diabetic comas and death.”
“Patients with type 1 and advanced type 2 diabetes try to keep their blood sugar levels under control with regular finger pricks and repeated insulin shots, a process that is painful and imprecise,” says John Buse, MD, PhD, co-senior author of the PNAS paper and director of the UNC Diabetes Care Center, adding: “Injecting the wrong amount of medication can lead to significant complications like blindness and limb amputations, or even more disastrous consequences such as diabetic comas and death.”
Consequently, the patch administration researchers have tried to remove more potential for human error by creating closed-loop systems that directly connect the devices that track blood sugar and administer insulin. However, these approaches involve mechanical sensors and pumps, with cumbersome and uncomfortable needle-tipped catheters that must be installed under the skin and replaced every few days.
Instead of inventing another completely man-made system, Dr. Gu and his colleagues chose to emulate the body’s natural insulin producers known as beta cells that act both as factories and warehouses, making and storing insulin in tiny sacs called vesicles. They also behave like alarm centers — as fore-noted, sensing blood sugar level increases in and signaling release of insulin into the bloodstream.
“We constructed artificial vesicles to perform these same functions by using two materials that could easily be found in nature,” explains Jiching Yu, a PNAS paper first author and PhD student in Dr. Gu’s lab.
The first material was hyaluronic acid or HA, a natural substance and an ingredient in many cosmetics. The second was 2-nitroimidazole or NI, an organic compound commonly used in diagnostics. The researchers connected the two materials to create a new molecule, with one water-loving or hydrophilic end that was and one that was water-fearing or hydrophobic. A mixture of these molecules is self-assembled into a vesicle, much like coalescing of oil droplets in water, with the hydrophobic ends pointing inward and the hydrophilic ends pointing outward.
The result was millions of bubble-like structures, each individual one some 100 times smaller than the width of a human hair. Into each of these vesicles, the researchers inserted a core of solid insulin and enzymes specially designed to sense glucose.
The researchers report that in lab experiments, as blood sugar levels increased excess glucose crowded into the artificial vesicles. The enzymes then converted the glucose into gluconic acid, consuming oxygen all the while. The resulting lack of oxygen or hypoxia made the hydrophobic NI molecules turn hydrophilic, causing the vesicles to rapidly fall apart and release controlled amounts of insulin into the bloodstream.
Once the researchers had their design for these intelligent insulin nanoparticles worked out, they needed to figure out a way to administer them to diabetes patients. Rather than relying on conventional large needles or catheters that have beleaguered previous approaches, they decided to incorporate the balls of sugar-sensing, insulin-releasing material into an array of tiny needles.

Dr. Gu created these microneedles using the same hyaluronic acid that was a chief ingredient of the nanoparticles, only in a more rigid form so that the tiny needles would be sufficiently stiff to pierce the skin. The scientists then arranged more than 100 of these microneedles on a thin silicon strip to create what looks like a tiny, painless version of a bed of nails. When the patch is placed on the skin, the microneedles penetrate the surface, tapping into blood flowing through capillaries just below.
The ability of this approach to control blood sugar levels was tested in a mouse model of type 1 diabetes. The researchers gave one set of mice a standard injection of insulin and measured the blood glucose levels, which dropped to normal but then quickly climbed back into the hyperglycemic range. By contrast, when the researchers treated another set of mice with their microneedle patch, they observed that blood glucose levels were brought under control within thirty minutes and stayed regulated for several hours.
The researchers additionally discovered that they could “tune” the patch to alter blood glucose levels only within a certain range by varying the dose of enzyme contained by each of the microneedles. They also found that the patch eliminated the hazards of insulin injections that can send blood sugar plummeting to dangerously low levels when administered too frequently.
“The hard part of diabetes care is not the insulin shots, or the blood sugar checks, or the diet but the fact that you have to do them all several times a day every day for the rest of your life,” says Dr. Buse, who is director of the North Carolina Translational and Clinical Sciences (NC TraCS) Institute and past president of the American Diabetes Association. “If we can get these patches to work in people, it will be a game changer.”
Because mice are less sensitive to insulin than humans, the researchers think that the blood sugar-stabilizing effects of the patch could last even longer when given to actual patients. Their eventual goal, Dr. Gu says, is to develop a smart insulin patch that patients would only have to change every few days.
The research was funded by a pilot grant from the NC TraCS Institute and a Pathway to Stop Diabetes Research Award from the American Diabetes Association.
Sources:
University of North Carolina
NC State University
Proceedings of the National Academy of Sciences
Image Credits:
University of North Carolina
Dr. Zhen Gu