In a recent study entitled “Roux-en-Y Gastric Bypass Surgery Induces Early Plasma Metabolomic and Lipidomic Alterations in Humans Associated with Diabetes Remission,” authors sought to determine whether Roux-en-Y gastric bypass induces early positive alterations to patients’ metabolic and lipidomic profile and if these changes are associated with their diabetic status. The study was published in the open-access journal PLOS One.
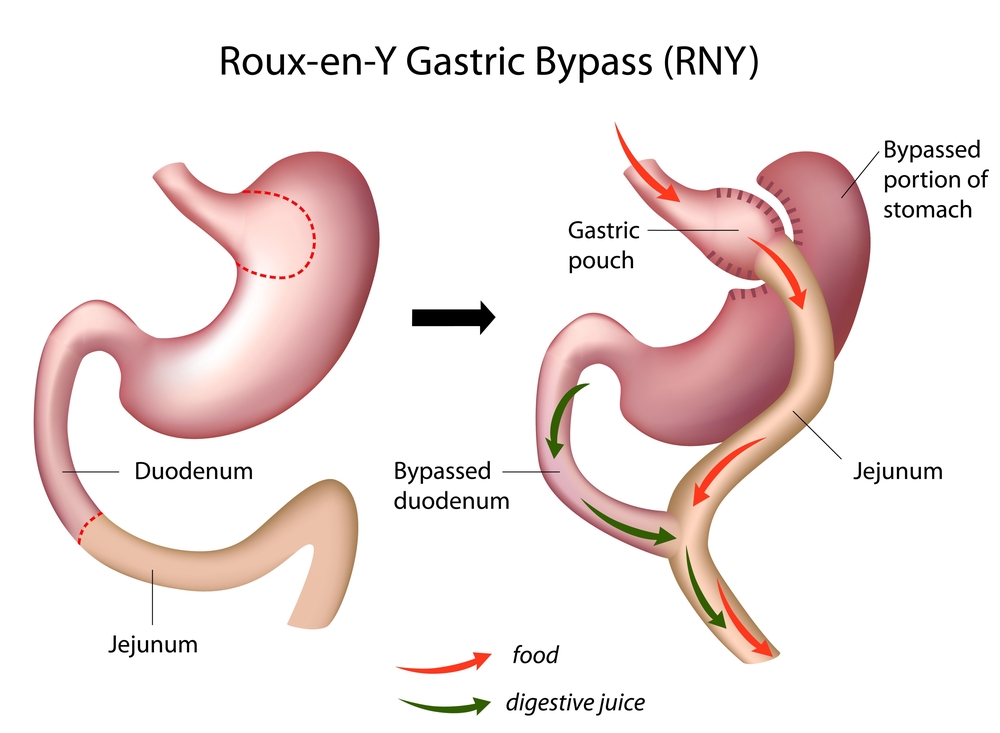
Roux-en-Y gastric bypass (RYGB) is an efficient strategy to maintain long term weight loss in morbidly obese patients. The technique consists of reducing the size of the stomach to that of a small pouch, about the size of an egg, and directly attaching the pouch to the small intestine. This reduces the amount of food a person can intake in each meal while it also decreases the amount of fat and calories absorbed. Not surprisingly, the surgery is associated with alterations in both metabolic and lipidomic (the total lipids profile) levels for patients, and has been increasingly recognized in improving patients’ glycemic control. Lacking in research, however, are insights into what early changes occur in both plasma metabolome and lipide after RYGB — key observations that would further highlight the surgery’s benefits in helping obese diabetics.
In this new study, a team of researchers performed a global metabolomics and lipidomics analysis of plasma samples retrieved from 16 insulin-resistant morbidly obese subjects (with 14 patients diagnosed with diabetes). The analysis was performed before RYGB surgery and then 4 and 42 days after. They identified 96 metabolites and 192 lipid molecular species in the plasma of patients. The team then compared patients’ metabolites and lipids results between time points and between patients who were in remission with those not in remission from diabetes (performed 2 years after surgery).
Researchers found that RYGB induced early changes in metabolomic and lipidomic profiles. Particularly, they observed that remission patients had higher levels of factors belonging to central metabolic pathways 4 days after surgery, which may contribute to an improvement in diabetes, when compared to those in non-remission. No differences were observed in metabolites and lipid species between both groups of patients 42 days after surgery.
The team recognizes some limitations to the study, such as the low number of patients and the lack of a non-diabetic control group that was not submitted to surgery. The findings report that gastric bypass does induce early positive alterations in the metabolome and lipids of patients with diabetes who undergo RYGB. However, these observations require further investigation in future studies with a larger patient cohort to validate and investigate the causes of potential diabetic remission after RYGB.