A novel device developed by a team of researchers at the University of Virginia (UVA) School of Medicine and the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), designed to automatically monitor and help regulate blood-sugar levels in persons with type 1 diabetes mellitus, will undergo final testing in two clinical trials beginning early this year.
Type 1 diabetes is a chronic autoimmune disorder characterized by the destruction of insulin-producing cells in the pancreas, resulting in loss of the ability to maintain blood glucose homeostasis. Approximately 1.25 million Americans, and an estimated 11 million to 22 million people worldwide, have type 1 diabetes, according to the U.S. Centers for Disease Control and Prevention.
If the “artificial pancreas” system performs in patients as well as scientists hope, results from these long-term clinical trials could lead the U.S. Food and Drug Administration (FDA) and other international regulatory groups to approve the device for use by people with type 1 diabetes.
With $12.6 million in support from the National Institute of Diabetes and Digestive and Kidney Diseases, part of the National Institutes of Health (NIH), the system will be tested in 240 patients with type 1 diabetes at nine sites in the United States and Europe through two six-month trials.
 The International Diabetes Closed-Loop trial is the first study of the technology, developed at UVA by a research team led by Boris Kovatchev, director of the UVA Center for Diabetes Technology. Participants will test the safety and effectiveness of the artificial pancreas for six months while going about their normal daily routines. The control-to-range artificial pancreas system technology has been further refined for clinical use and is now licensed to Charlottesville, Virginia-based startup company TypeZero Technologies. The investigators will compare the artificial pancreas’ performance to that of a standard insulin pump on two key measures: how well blood sugar levels are controlled, and whether the risk of hypoglycemia is reduced.
The International Diabetes Closed-Loop trial is the first study of the technology, developed at UVA by a research team led by Boris Kovatchev, director of the UVA Center for Diabetes Technology. Participants will test the safety and effectiveness of the artificial pancreas for six months while going about their normal daily routines. The control-to-range artificial pancreas system technology has been further refined for clinical use and is now licensed to Charlottesville, Virginia-based startup company TypeZero Technologies. The investigators will compare the artificial pancreas’ performance to that of a standard insulin pump on two key measures: how well blood sugar levels are controlled, and whether the risk of hypoglycemia is reduced.
 The second study will follow 180 patients who completed the first study for an additional six months to test the advanced adaptive control algorithm developed by the research team, led by co-principal investigator and engineering lead on the project Dr. Frank Doyle, John A. & Elizabeth S. Armstrong Professor of Engineering & Applied Sciences at the Paulson School, to test whether it further improves control of blood-sugar levels.
The second study will follow 180 patients who completed the first study for an additional six months to test the advanced adaptive control algorithm developed by the research team, led by co-principal investigator and engineering lead on the project Dr. Frank Doyle, John A. & Elizabeth S. Armstrong Professor of Engineering & Applied Sciences at the Paulson School, to test whether it further improves control of blood-sugar levels.
The Harvard scientists’ system is based on zone model-predictive control (zone MPC), a strategy originally developed by Dr. Doyle and colleagues and described in a paper published in 1996. Rather than regulating glucose levels to a specific point in the same way that a home thermostat keeps room temperature at a precise setting, zone MPC defines an acceptable range for an individual’s glucose levels and controls variables to stay within it.
In conventional treatment, diabetics must self-monitor their blood-glucose levels, count carbohydrates, and when necessary, administer doses of insulin via either needle injections or an insulin infusion pump — techniques that result in only about 25 percent of diabetics meeting the standard for good glycemic control. Failure to maintain proper blood glucose levels by managing insulin is associated with long-term complications that include heart disease, kidney disease, and neuropathy. Poor blood-glucose control has also been shown to cause low energy, mood swings, and cognitive disruptions on a daily basis.
Through a match of control engineering with medical practice and behavioral science, the artificial pancreas system is designed to supply appropriate levels of insulin by not only reacting to changes in the body, but accurately predicting future blood glucose levels.
“To be ultimately successful as an optimal treatment for diabetes, the artificial pancreas needs to prove its safety and efficacy in long-term pivotal trials in the patient’s natural environment,” said Dr. Kovatchev in a UVA press release. “Our foremost goal is to establish a new diabetes treatment paradigm: the artificial pancreas is not a single-function device; it is an adaptable, wearable network surrounding the patient in a digital treatment ecosystem.”
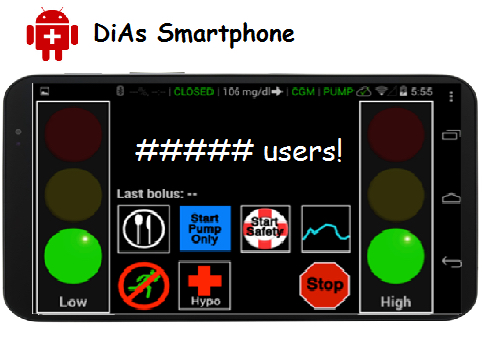
Ideally, the artificial pancreas will eliminate the need for people with type 1 diabetes to stick their fingers multiple times daily to check blood-sugar levels and to inject insulin manually. The artificial pancreas is, of course, not a replica organ, but rather an automated insulin delivery system designed to mimic a healthy person’s glucose-regulating function. The closed-loop system consists of an insulin pump, a continuous glucose monitor placed under the user’s skin, and advanced control algorithm software embedded in a smartphone that provides the engineering brains, and is designed to oversee and adjust the amount of insulin delivered by pump based on a range of variables, including meals consumed, physical activity, sleep, stress, and metabolism.
At the center of the artificial pancreas platform, known as “InControl,” is a reconfigured smartphone running advanced algorithms and that is linked wirelessly to a blood-sugar monitor and an insulin pump worn by the patient, as well as to a remote-monitoring site. People with the artificial pancreas can also access assistance via telemedicine.
“The idea is that this can lead to an improved quality of life for individuals with this disease — not a solution to diabetes, but a means to really extend the quality of their healthful living,” said Dr. Doyle.
“The biggest challenge in the design of the artificial pancreas is the inherent uncertainty in the human body,” he added. “Day to day, hour to hour, the various stresses that impact the human body change the way it responds to insulin-controlling glucose. Physical stresses, anxiety, hormonal swings will all change that balance. To be able to control for those factors we need to see longer intervals of data. This is the first trial where we’ll be looking at multi-month intervals of time with cohorts of subjects where we can actually see a long enough window to learn those patterns, to adapt and fine-tune the algorithms, and to improve the overall level of glucose control.”
 Dr. Doyle and SEAS senior researcher Dr. Eyal Dassau, who were collaborators at the University of California, Santa Barbara, before joining Harvard in the fall of 2015, are also part of a team working on a pediatric version of the artificial pancreas system, as well as an implantable version of the device.
Dr. Doyle and SEAS senior researcher Dr. Eyal Dassau, who were collaborators at the University of California, Santa Barbara, before joining Harvard in the fall of 2015, are also part of a team working on a pediatric version of the artificial pancreas system, as well as an implantable version of the device.
Persons interested in participating in one of the clinical trials can find more information through: med.virginia.edu/diabetes-technology/contact/
Along with UVA in Charlottesville, Virginia, and Harvard University in Massachusetts, the artificial pancreas will be tested at seven additional sites: the Icahn School of Medicine at Mount Sinai in New York; the Mayo Clinic in Rochester, Minnesota; the University of Colorado; Stanford University in California; the University of Montpellier in France; the University of Padova in Italy; and the Academic Medical Center at the University of Amsterdam in The Netherlands.
The NIH/NIDDK grant is No. UC4DK108483.
Sources:
University of Virginia (UVA) School of Medicine
Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS)
National Institute of Diabetes and Digestive and Kidney Diseases
National Institutes of Health
U.S. Centers for Disease Control and Prevention
TypeZero Technologies