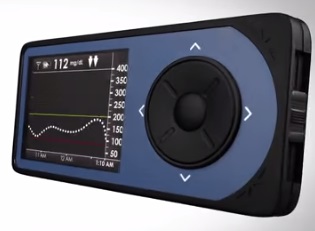
 The U.S. Food and Drug Administration (FDA) released an informational overview for the recently approved Dexcom G4 PLATINUM Continuous Glucose Monitoring (CGM) System for the management of diabetes. The system, which was developed by Dexcom, Inc. to identify episodes of either high or low blood glucose, was approved by the FDA on October 21st and will now be sold in the United States.
The U.S. Food and Drug Administration (FDA) released an informational overview for the recently approved Dexcom G4 PLATINUM Continuous Glucose Monitoring (CGM) System for the management of diabetes. The system, which was developed by Dexcom, Inc. to identify episodes of either high or low blood glucose, was approved by the FDA on October 21st and will now be sold in the United States.
The monitoring system is worn externally and is able to continuously measure and display glucose values measured within the fluid between the body’s cells through an internal sensor placed under the skin of the abdomen. Its second component, the transmitter, then provides reports on the patient’s interstitial glucose values every five minutes, which are sent to the hand-held receiver. The main purpose of the device is to offer data on glucose trends and patterns in order to target abnormalities.
“The Dexcom G4 PLATINUM CGM System aids in detecting episodes of high blood glucose (hyperglycemia) and low blood glucose (hypoglycemia),” the FDA stated in their overview. “The system helps with both short-term and long-term therapy adjustments, which may minimize these highs and lows in blood glucose. Interpreting the Dexcom G4 PLATINUM CGM System results should be based on the trends and patterns seen with several consecutive readings over time.”
However, the FDA also notes that using the system does not replace the use of a standard home blood glucose meter to obtain blood sugar information. The Dexcom G4 PLATINUM CGM System is designed to serve as an additional tool for providing complementary glucose information. The use of the system may enable patients to avoid potential complications due to high or low levels of glucose. In addition, for long-term use, it may also help physicians adjust patients’ treatment plans according to the pattern of their glucose levels.
The FDA recommends that “the Dexcom G4 PLATINUM CGM System sensor, transmitter, and receiver should be removed before Magnetic Resonance Imaging (MRI), Computed Tomography (CT) scan, or diathermy treatment,” since it has not been tested during these treatments or exams. However, “the magnetic fields and heat could damage the System so that it might not display sensor glucose readings or provide alerts; and a low or high interstitial glucose value may be missed.”
Patients should also be aware that medication, even when prescribed, may affect the data, since drugs such as acetaminophen can falsely raise glucose sensor readings. However, the deviation and the amount of compound in the body are dependent on the patient’s body and system.
For further information, watch this video on the Dexcom G4 PLATINUM Continuous Glucose Monitoring (CGM) System: